|
Each kind of chemical bond - covalent,
ionic, hydrogen, polar, and so on - is created by a unique kind of electron behavior that, in turn, appears as a uniquely shaped electron density cloud.
This essay describes the characteristic electron density patterns of covalent bonds and ionic bonds.
The patterns are useful because they help us understand the different nature of these bonds. They are also useful because, whenever we have adequate electron density, we can use these patterns to assign bond types and locations.
(And, as added bonuses, we can often assign
bond orders and detect electron delocalization.)
Covalent vs. Ionic
Covalent bonds are associated with a build-up of electron
density between the bonded atoms. Ionic bonds do not involve electron sharing, and do not show this build-up.
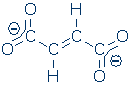
The following salt, disodium fumarate, is held together
by a combination of covalent and ionic bonds, so its electron density
cloud should reveal both of these characteristic patterns.

Isodensity surfaces representing different regions of the electron density cloud (core, bonding, and molecular) of disodium fumarate are shown below. As you inspect each surface, pay attention
to these questions (clicking on a surface will give you a larger image):
 |
- What part of the electron density cloud am I looking at? (Is the electron density on this surface relatively high, medium, or low?)
- Where are the atoms located?
- Does the surface provide any information about bond location and bond type? (Does it show a build-up of electron density between atoms?)
|
 |
| 0.4 |

core (very hi r (rho)) regions
- no connectivity info |
| 0.3 |

bonding (high r (rho)) regions
- C=O + C=C connectivity info |
| 0.2 |

bonding (medium-high r (rho))
regions - all covalent connectivity info |
| 0.1 |

bonding (medium r (rho)) regions
- all covalent connectivity info |
| 0.01 |

molecule (low r (rho)) region
- no connectivity info |
0.002
SIZE |

molecular (very low r (rho))
region - no connectivity info |
|
The surfaces that mark very high (≥ 0.4) and very low (≤ 0.01) electron density are not
informative. The former are located in atom cores, while the latter are located on the molecule's outer fringes. Neither region is affected by electron sharing.
The useful surfaces are those of medium (0.1) to medium-high (0.3) electron density. Some of these surfaces enclose all of the covalent bonds (confirming that electron density builds up between covalently bonded atoms) and show covalent connectivity. Others only enclose selected bonds (see below). None of the surfaces enclose ionic bonds because electron density is much lower in these regions (<< 0.1).
These patterns apply to all molecules. Electron density builds up substantially (> 0.05) in all covalent bonding regions, and is even higher in regions that correspond to multiple bonds (see below). Therefore, inspection of several isodensity surfaces can reveal covalent bonds and ionic bonds alike.
Bonus - Bond orders too!
In many cases, you can also obtain bond order information
from isodensity surfaces. Compare the following surfaces
of disodium fumarate (duplicated from above):
 |
| 0.3 |

bonding (high r (rho)) regions
- only multiple bonds detected |
| 0.1 |

bonding (medium r (rho)) regions
- all covalent bonds detected |
|
The degree of electron density build-up depends on
the bond order. Electron density builds up more (≥ 0.3) where there are multiple bonds, and to a lesser degree (≥ 0.1) where there are only single bonds.
Unfortunately, using isodensity surfaces to assign bond orders is
a tricky business. A single surface (for example, 0.1) may not show much, while another
surface (0.3) might be very revealing. It is also difficult to anticipate
what values of r should be looked at, so it is necessary to study a range (0.1 - 0.3 is usually sufficient).
Bottom-line: You can use electron density to compare
bond orders between the same kind of atoms, say, one CO bond with another
CO bond (but not CO vs. CN). If you strike it lucky and find an
isodensity surface that shows you two bonds are different, then
they really are! But be cautious. It might be hard to say how
different they are. It is always best to combine electron density information with other
information, like bond distance, bond angle, and electrostatic potential.
Extra special bonus - Delocalized bonds!!
If you take one more look at the 0.3 isodensity surface (duplicated below),
you will see that it has the same shape in all four CO bonding
regions. The other isodensity surfaces also look identical in these regions. Taken together, these surfaces show that the electron density cloud is roughly the same
in each CO bonding region, and all
of the CO bonds are equivalent.
 |
| 0.3 |

bonding (high r (rho)) regions
- only multiple bonds detected |
|
It is impossible to draw a Lewis structure that contains
4 identical CO bonds and also gives every atom an ideal gas electron
configuration (Lewis octet). Therefore, we must conclude that the fumarate
dianion is a resonance hybrid and the CO bonds are delocalized partial
double bonds.
This finding demonstrates another interesting aspect of electron density. Unlike Lewis' theory, which relies on an exceedingly simplistic view of electron sharing, quantum mechanics (and Mother Nature) admit a continuous range of electron-sharing possibilities and the entire range is reveals itself through the electron density cloud.
Review problems
#1. Use these isodensity surfaces to answer
questions about LiKSO4 (click
on each surface for a larger image).
a. How many ions are present in this compound?
answer
b. These surfaces establish the connectivity
of KLiSO4. Draw its Lewis structure.
answer
#2. Use these isodensity surfaces to answer
questions about N,N-dimethylimidazolium chloride, C5H9ClN2
(click on each surface for a larger image).
a. How many ions are present in this compound?
answer
b. These surfaces establish the connectivity
of this compound and some of its bond orders. Draw its Lewis structure.
Note: if this compound looks like a resonance hybrid, draw all important
resonance forms.
answer
|



