Germ Warfare
Reed biologists use a virus to attack a deadly strain of E. coli.
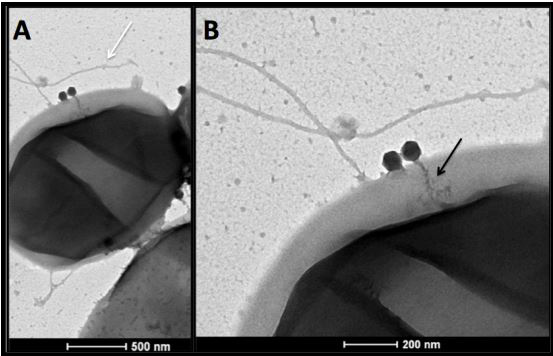
The virus looks like a spaceship. Spindly tail fibers jut out from its neck like landing supports, while its icosahedral head adds to the otherworldly appearance. But this microscopic life form is not science fiction—it is a real entity, swimming in the petri dish I hold in my hand. Its name is PDX, and one day it could save thousands of lives from fatal foodborne illness.
Discovered in the lab of Prof. Jay Mellies [biology], PDX has a single mission: it is a bacteriophage (“phage” for short), a virus that only attacks bacteria, and it specializes in a deadly strain of bacteria known as pathogenic E. coli. PDX hijacks the natural systems of pathogenic E. coli in order to replicate, killing its host by bursting the infected bacterial cell apart. My job this summer is trying to locate the structural weak spot on the bacterium’s armor that the phage exploits.
Thanks to the Helen Stafford Research Fellowship, I have been able to work in Prof. Mellies’ lab full-time over the summer as a rising junior. I am studying PDX’s host range—how many strains of bacteria it can target and kill—and the elusive receptor through which PDX adsorbs into and infects its bacterial hosts.
As the midsummer sun glares down upon the world outside, the refrigerated lab continues to protect me from the heat as I collect data from the day before in shaded comfort. In a typical day, I might inspect 30 petri dishes of E. coli to see if they are speckled with plaques—circular regions of no growth that indicates the presence and replication of phage. I also delve into the biochemical side of my research.
The beauty of summer research is that I get to apply knowledge from my classes while building upon new skills, such as learning how to sift through dozens of academic papers and stitch together my own protocols for hunting down the phage-host receptor. Jay has been a phenomenal teacher, guiding me when I get stuck and overall supervising the research so that it never derails, but he has also encouraged me to take the lead in directing the future of our phage research.
“Research allows students to become independent working in laboratory and design experiments with a faculty mentor, and carry those out and to understand how scientific information is gathered,” he says. “I think the greater benefit is teamwork.”
Most recently, I have learned how to run SDS-PAGE, a form of gel electrophoresis that denatures proteins and carbohydrate protrusions of E. coli that are often responsible for pathogenicity (lipopolysaccharides or LPS). This separates the molecules by weight across the gel so that the components can be stained and compared visually for similarities and differences in composition. Essentially, the process is like fish from different ponds swimming through a net with holes in it—the smaller fish swim farther in a given amount of time. Comparing data from bacteria that are susceptible to PDX to those that are not seems to suggest—initially, at least—that the receptor is not LPS, but instead an outer membrane protein.
As an intended biology-anthropology major, I hope to transform my summer research into an ongoing project and to pursue this line of research for my thesis. These tiny phage have the potential for massive impact against the ever-growing onslaught of antibiotic-resistant bacteria, and PDX itself could one day be a component of treatment to combat illness. By fully understanding this chink in the bacteria’s armor, PDX could be transformed from an alien virus to a life-saving therapy in this microbial war of the worlds.
Tags: Academics, Research, Health/Wellness, Cool Projects, Editor's Picks