Current Projects
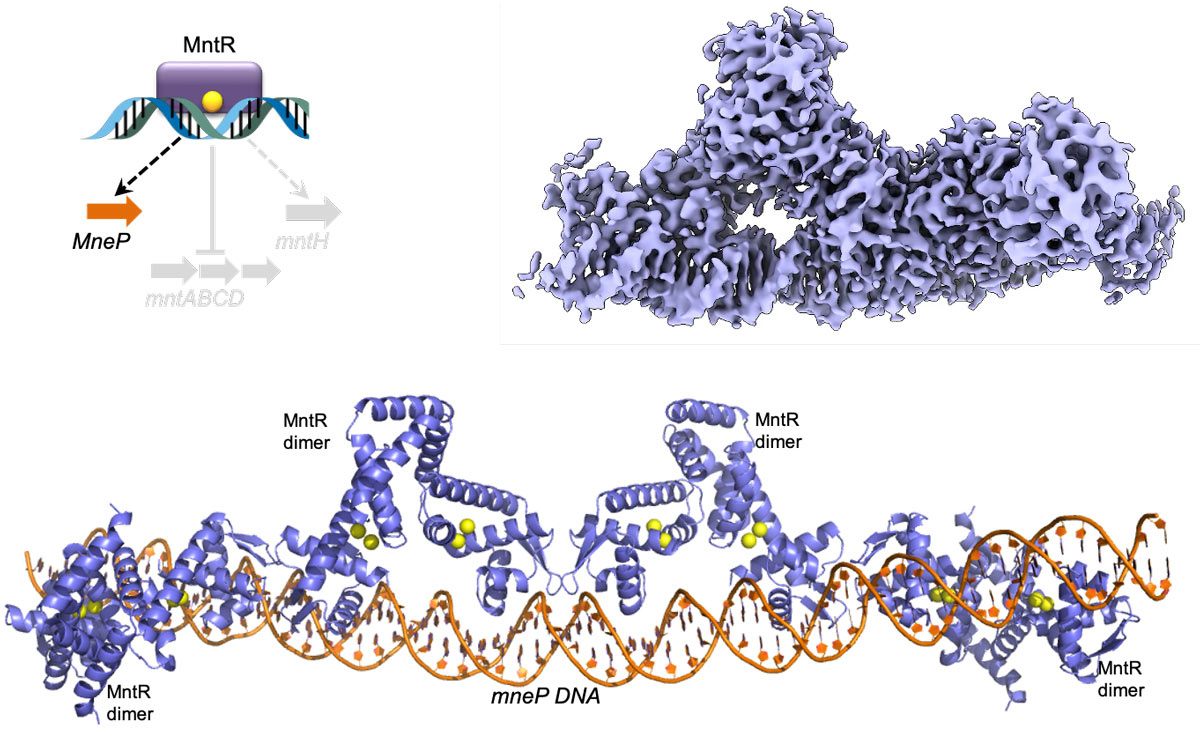
The dual role of MntR: A central regulator of bacterial Mn homeostasis
MntR is a metalloregulator and a transcription factor natively expressed in Bacillus subtilis. The transcription factor is unique in its ability to bifunctionally regulate the expression of both Mn importers (MntH and MntABCD) as well as Mn exporters (MneP and MneS) in response to the intracellular concentration of free Mn ions. We are using cryo-EM and in vitro assays such as mass photometry to investigate the structural basis of transcription activation and repression by MntR.
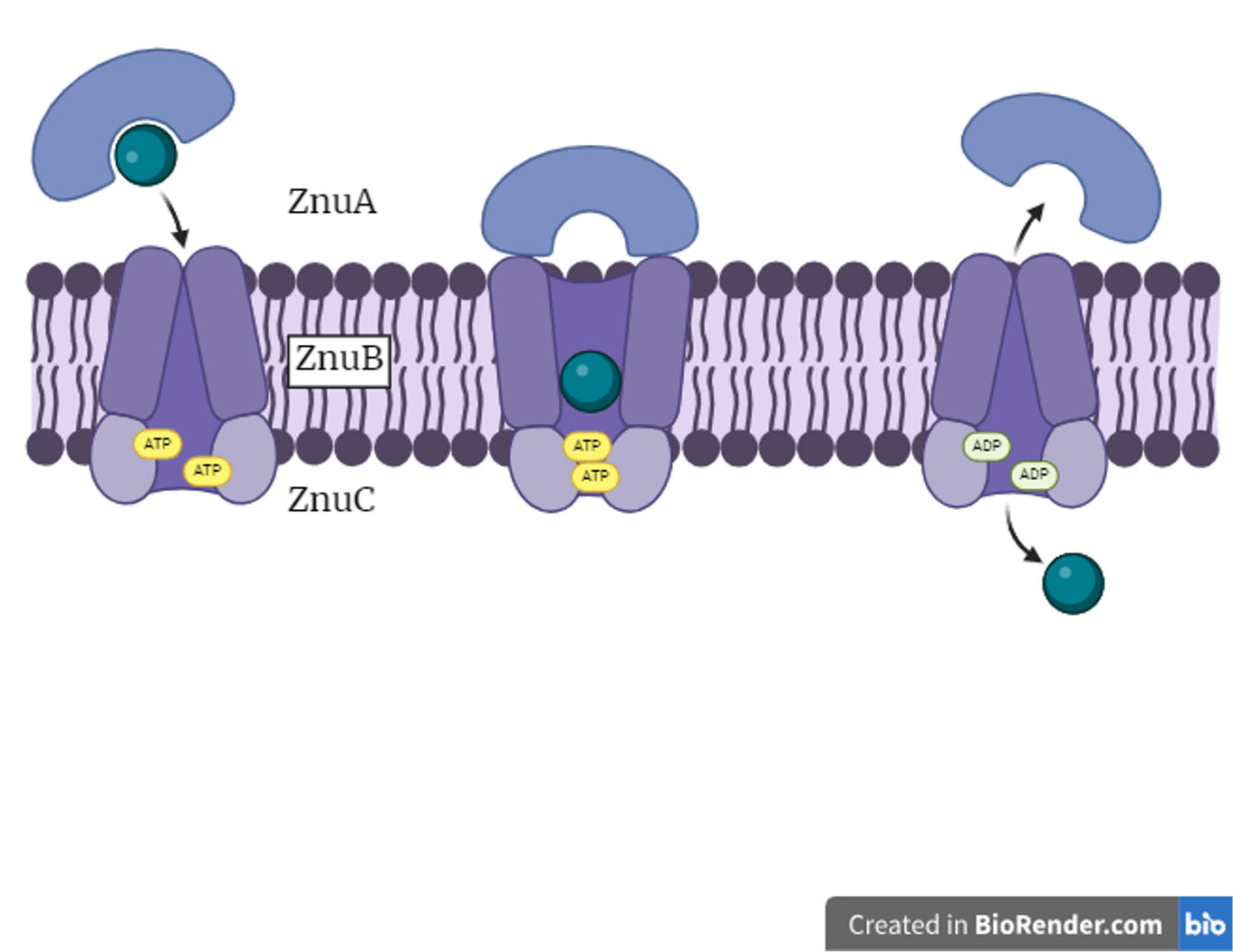
“ABC” of bacterial metal ion acquisition
The pico-to-nanomolar affinity of ATP binding cassette (ABC) transporters for their cognate metal ion allows bacteria to access trace amounts of the metal ion in a host microenvironment. We are studying the different conformations of the ABC transporter complexes specific for Mn and Zn from capture to translocation of the cognate metal ion into the cell.
Cation diffusion facilitator proteins are essential for the prevention of fatal manganese intoxication
Bacterial survival hinges on dynamic interactions with the environment, facilitated by transmembrane transport systems such as MneP and MneS in B. subtilis. These proteins, as part of the cation diffusion facilitator (CDF) family, regulate the efflux of trace metal ions, Mn and Fe. Their expression, governed by MntR, ensures proper metal levels for biological processes and prevents manganese intoxication. Our goal is to understand the proton motive force (PMF) driven mechanism of Mn ion export via the MneP and MneS exporters
Structural & spectroscopic investigation of the dual role of TerC in biological tellurium detoxification and Mn(II) export
A collaborative project with Dr. Kelly N. Chacón in the chemistry department at Reed College. The Chacón lab is focused on understanding the function of the terZABCDEF operon that has been identified in every tellurium-resistant bacterium. Recent findings suggest that TerC homologs in B. subtilis possess manganese efflux capabilities, albeit weaker compared to the primary (MneP) and secondary (MneS) Mn exporters. We collaborate with the Chacón lab to structurally and functionally characterize the TerC protein from E.coli and study its specificity for different transition metal ions.
[1] Szklarczyk et. al. (2023). Nucleic Acids Res. Jan 6;51(D1):D638-D646 doi: 10.1093/nar/gkac1000
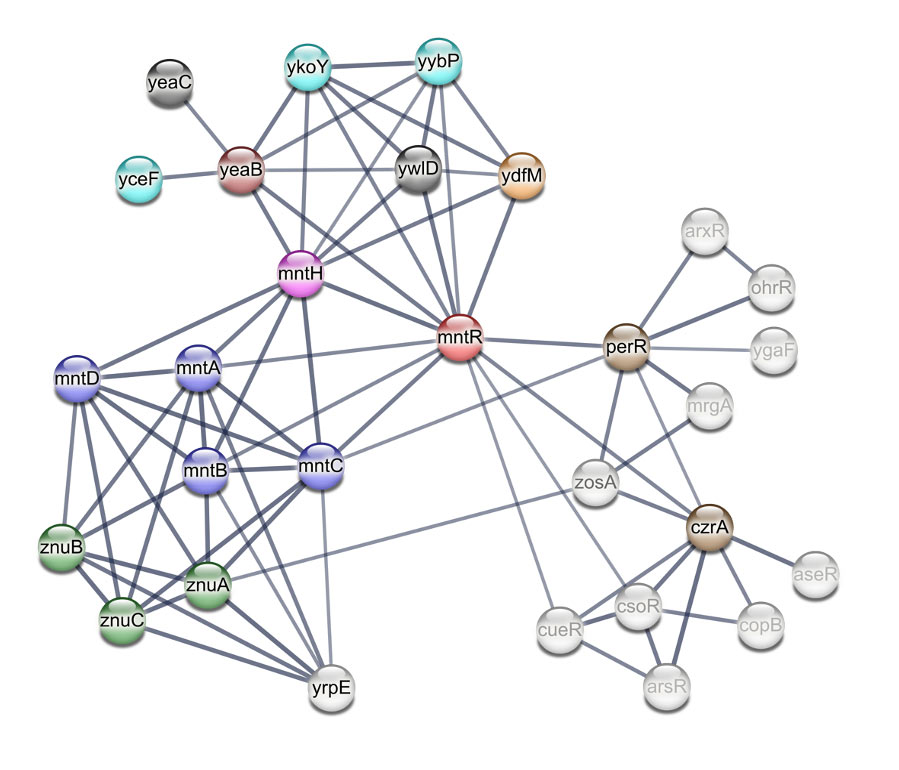
Networking in metal ion homeostasis: identifying new players involved
Protein networks regulating Mn homeostasis in bacteria do not operate independently but rather are interconnected with protein networks managing other metal ions like iron. Our lab is collaborating with Dr. Anna Ritz, a computational biologist at Reed College in exploring all the players involved in Mn homeostasis in B. subtilis. It’s a computationally driven project where we have used motif finding, transcriptomic analysis, and experimentally derived interactomes that highlight thousands of protein-protein interactions (PPI) from B. subtilis, to identify other proteins involved in Mn homeostasis, and to find links to other systems that sense Fe and Zn or reactive oxygen species (ROS).