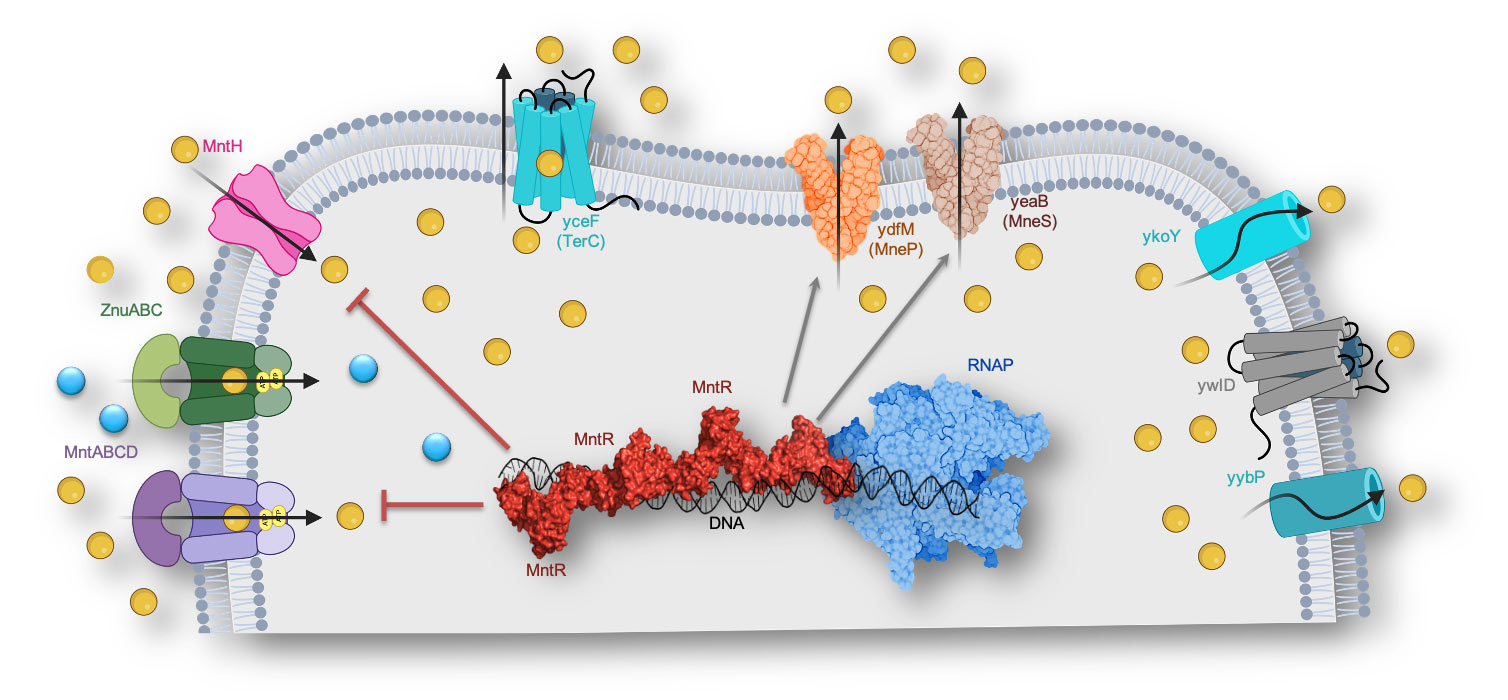
Comprehensive understanding of bacterial metal ion homeostasis
Metal ions are fundamental to numerous vital cellular functions and cannot be created or destroyed. Transition metals like manganese and zinc, with their strong Lewis acid properties, are crucial as catalytic and structural cofactors in a variety of metalloproteins. Our research focuses on understanding the mechanisms of proteins involved in bacterial Mn and Zn homeostasis, essential for cellular survival and virulence. Manganese is universally vital in bacteria for resisting oxidative stress caused by reactive oxygen species (ROS) from both endogenous metabolism and extracellular oxidants. However, excessive transition metals can be toxic, causing mis-metalation of metalloenzymes and transcription factors. To counteract this, bacteria have evolved metalloregulatory proteins that bind specific metal ions and regulate the expression of metal-ion influx (importer) and efflux (exporter) transporter proteins. These proteins ensure that the intracellular concentrations of metal ions are finely balanced to prevent toxicity while maintaining necessary levels for bacterial function.
Our lab focuses on Mn and Zn homeostasis in the gram-positive bacteria Bacillus subtilis. Using techniques like single-particle cryogenic electron microscopy (cryo-EM) and X-ray crystallography, we aim to determine the atomic resolution structures of proteins involved in different stages of Mn and Zn homeostasis. Additionally, we develop functional assays to better understand each protein’s role. This integrated approach will elucidate the mechanisms of metal ion homeostasis and has significant implications for designing antibiotic alternatives to combat bacterial infections.