Investigating the Transition from Proliferation toward Differentiation in the Vertebrate Visual System
I’m fascinated by
 Retinal Neurogenesis
Retinal Neurogenesis
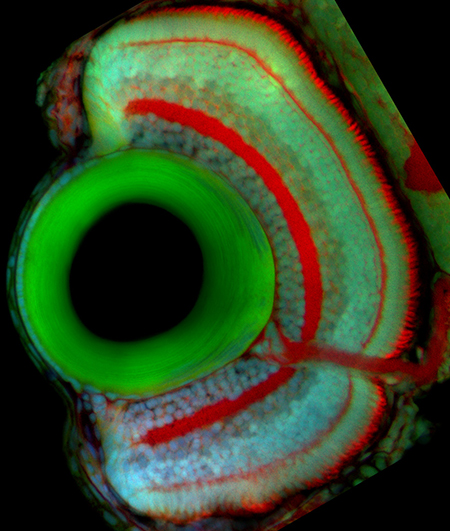
Proliferation, differentiation, and death are precisely coordinated throughout development. Cancers, developmental defects, and degenerative disorders are examples of development gone awry and can result from defects in the transition from proliferation to differentiation. To learn how tissues of the correct size and composition are formed, my lab studies how progenitor cells transition from proliferation toward differentiation during eye growth and development. In most vertebrates, neurogenesis occurs first in the central retina (early neurogenesis) and followed by neurogenesis in the peripheral retina (late neurogenesis). Both types of neurogenesis rely on a successful transition from proliferation to differentiation, but occur in distinct contexts. By comparing the two processes, we hope to tease apart the relative importance of intrinsic and extrinsic cues for the two types of neurogenesis.
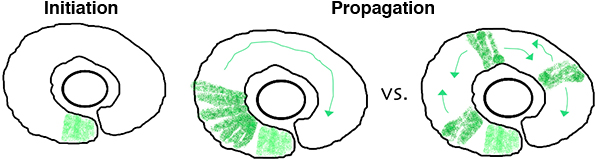 In the zebrafish retina, central neurogenesis follows a two-step process. First, a ventral-nasal patch of cells express neurogenic/differentiation genes, initiating neurogenesis. Next, a subset of proliferating neuronal progenitors stop dividing, turn on neurogenic genes, propagating the neurogenic wave throughout the retina. Although previous work has implicated cues from the midline, such as Shh, in these processes, many open questions remain. How are cell cycle exit and neurogenic decisions coordinated? What precise path does the neurogenic wave take? What is the relative importance of cell-cell interactions versus cell-intrinsic programs during
In the zebrafish retina, central neurogenesis follows a two-step process. First, a ventral-nasal patch of cells express neurogenic/differentiation genes, initiating neurogenesis. Next, a subset of proliferating neuronal progenitors stop dividing, turn on neurogenic genes, propagating the neurogenic wave throughout the retina. Although previous work has implicated cues from the midline, such as Shh, in these processes, many open questions remain. How are cell cycle exit and neurogenic decisions coordinated? What precise path does the neurogenic wave take? What is the relative importance of cell-cell interactions versus cell-intrinsic programs during
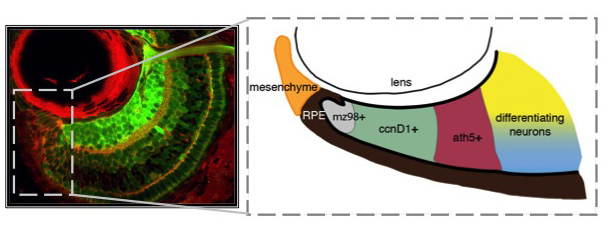 Unlike you and me, fish grow from the day they're born until the day they die. Throughout their bodies, stem cells are sequestered in specialized niches. Zebrafish have at least 17 distinct stem cell niches in their central nervous system alone, including a retinal stem cell niche termed the ciliary marginal zone (CMZ). The CMZ is located at the periphery of the retina, and it is this group of cells that contribute to eye growth and late neurogenesis. The CMZ is organized with the youngest and least determined stem-like cells nearest the periphery (mz98+), proliferative progenitors (ccnD1+) and committed precursors (ath5+) in the middle, and differentiating neurons abutting mature neurons in the central retina. By studying how cells within the CMZ behave, it is our goal to determine how specific extrinsically modulated pathways, including Hedgehog, TGF-β, Notch, and Retinoic Acid control cell cycle exit and differentiation of neuronal stem cells.
Unlike you and me, fish grow from the day they're born until the day they die. Throughout their bodies, stem cells are sequestered in specialized niches. Zebrafish have at least 17 distinct stem cell niches in their central nervous system alone, including a retinal stem cell niche termed the ciliary marginal zone (CMZ). The CMZ is located at the periphery of the retina, and it is this group of cells that contribute to eye growth and late neurogenesis. The CMZ is organized with the youngest and least determined stem-like cells nearest the periphery (mz98+), proliferative progenitors (ccnD1+) and committed precursors (ath5+) in the middle, and differentiating neurons abutting mature neurons in the central retina. By studying how cells within the CMZ behave, it is our goal to determine how specific extrinsically modulated pathways, including Hedgehog, TGF-β, Notch, and Retinoic Acid control cell cycle exit and differentiation of neuronal stem cells.
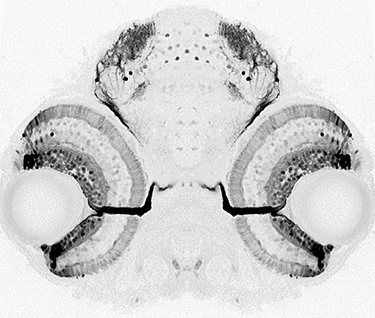
What are the mechanisms that promote a continuous supply of new neurons and prevent tissue overgrowth? Not only is this question important for understanding how fish eyes grow and develop,
Another challenge that life-long retinal growth presents is that not only the eye but also its target tissue in the brain, the optic tectum, must grow synchronously. Both the retina and the tectum must add a precise number of cells so that the fish can accurately represent the information gathered by the eye in the brain. In collaboration with Maté Varga and Steve Wilson’s group at the University College London, we are exploring mechanisms that enable coordinated growth between the retina and the